Abstract
Purpose:
Non-myeloablative allogeneic hematopoietic cell transplantation (NMA-HCT) can provide prolonged remissions in patients with advanced B-cell lymphoma (B-NHL) via the graft versus lymphoma (GVL) effect, though inferior results are seen in patients with chemoresistant, bulky, or aggressive disease. Radioimmunotherapy (RIT) can safely induce responses in B-NHL with little non-hematologic toxicity. Initial results of this approach demonstrated early safety and disease control (Blood, 2011) but with short follow up. Here we report the long-term outcomes of patients treated on this study with specific emphasis on patients achieving early remissions.
Patients and Methods:
Eligible patients with persistent CD20+ B-NHL or CLL, with HLA-matched related or unrelated donors, and adequate organ function, underwent NMA-HCT. Patients were not excluded based on cytopenia or marrow involvement with lymphoma. Conditioning included 0.4 mCi/kg (max 32mCi) 90Y-ibritumomab tiuxetan (day-14), fludarabine (days -7 to -5) and 2 Gy total body irradiation (day 0) followed by transplantation from HLA-matched related or unrelated donors along with post-grafting immunosuppression with cyclosporine and mycophenolate mofetil. Long-term follow up was updated through July 1, 2017.
Results:
We accrued 40 patients to this prospective trial. Baseline features included: median age 58 years (range 29-69), median prior regimens = 6 (range 3 to 12), chemosensitive disease = 6 (15%), CR pretransplant = 0 (0%), bulk >5 cm = 17 cm (range 5.2-18.6cm, 43%), >25% bone marrow involvement with B-NHL = 10 (25%, range of marrow involvement 40-95%), comorbidity score ≥1 = 35 (85%), median comorbiditiy score 3 (range 0-10), pretransplant IPI ≥3 = 21 (53%). Histologies included: indolent (18; CLL/SLL-10, follicular lymphoma-6, hairy cell leukemia-1, and marginal zone lymphoma-1), Diffuse large B-cell (DLBCL, 14), and mantle cell lymphoma (MCL, 8). Patients received HCT from 15 matched related or from 25 matched unrelated donors.
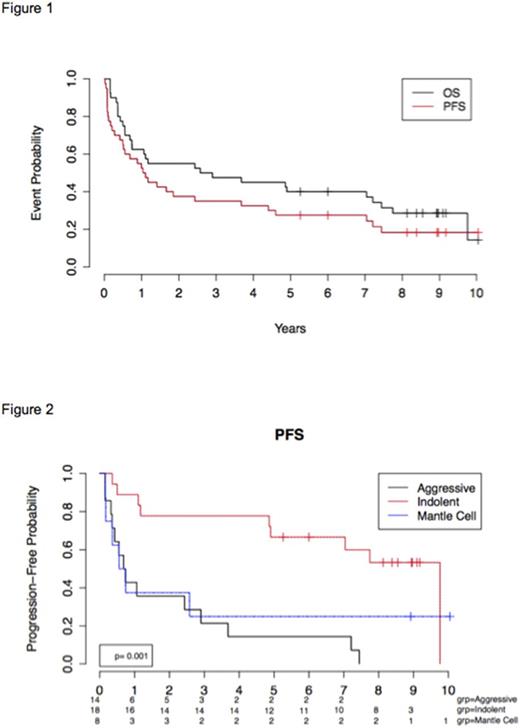
Eleven of 40 patients are alive with a median follow up of 9 years (range 5.3-10.2). There were 14 (35%) deaths due to disease progression, and 14 (35%) deaths attributed to complications from HSCT. One patient died of a Merkel cell carcinoma. The median progression-free and overall survival of the entire group was 1.1 years and 2.9 years, respectively (Figure 1). The best outcomes were observed in patients with indolent histology with an estimated 5-year PFS and OS of 44% and 67% respectively (Figure 2). Early CR/PR was achieved by 7/8 (83%) patients with indolent lymphoma, 4/1 (36%) with DLBCL, and 2/2 (50%) with MCL. Long-term PFS was observed in 7 of 15 of patients with indolent NHL and 2 of 4 MCL with early remission. None of the patients with DLBCL were long-term disease free survivors regardless of early remission status. No late complications attributable to RIT (such as secondary myeloid malignancies) were observed in any patient.
Conclusions:
Long-term disease control of high-risk B-NHL not in CR who underwent NMA-HCT with 90Y-ibritumomab tiuxetan conditioning varied by histology. 90Y-ibritumomab tiuxetan based allografting induced high-early response rates and favorable long-term outcomes in patients with indolent histologies likely based on both the efficacy of RIT and GVL effect in this population. Only early MCL responders to RIT had long term PFS, and none of the DLBCL patients survived regardless of response. Improved strategies are needed for these populations. Our current study escalates the dose of 90Y-ibritumomab tiuxetan prior to NMA-HCT for DLBCL for this purpose.
Flowers: Pharmacyclics: Consultancy. Maloney: Juno Theraapeutics: Other: Advisory board, Patents & Royalties, Research Funding; Roche/Genetech: Other: Advisory board; Celgene: Other: Advisory board; Kite Pharmaceuticals: Other: Advisory board. Press: Roche: Honoraria, Research Funding; BMS: Honoraria; Bayer: Consultancy. Gopal: Seattle Genetics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal